BIOBANKING
Quality Management System
Quality Management System
About
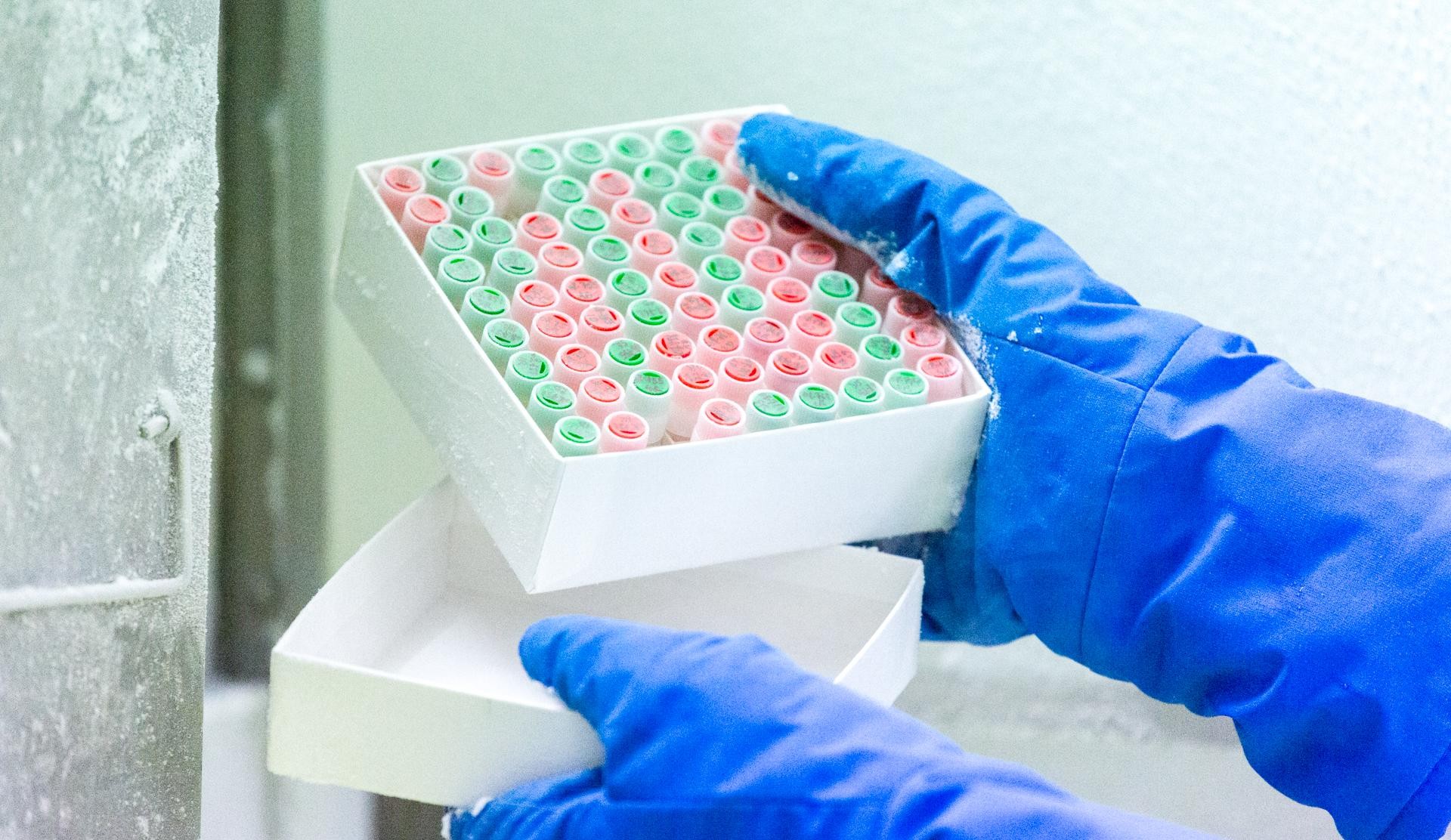
The intrinsic quality of biological resources (samples and associated data) is at the heart of Biobank Côte d’Azur’s priorities. It represents the enhancement of collections conserved for research purposes.
The Biobank Côte d’Azur implements and uses standardized, optimized techniques and procedures for the management, collection, reception, preparation, analysis, conservation and provision of biological resources (biological material and associated data). The aim is to provide users with reproducible, reliable, consistent and optimal quality samples.

Quality Management System
QMS policy
Since 2010, the Biobank Côte d’Azur has been committed to an optimized quality approach and is certified according to the NF S96-900 standard (French national standard), which specifies the requirements for the management system of a Biological Resources Centre (BRC) and the quality of biological resources.
The Biobank Côte d’Azur, integrated into the clinical and experimental pathology laboratory (LPCE) of the Nice University Hospital, is accredited by this laboratory.
It has been accredited by COFRAC according to ISO 15189 for its activities in Pathology ACP (Anatomy Cytology Pathology, Immuno-Histochemistry) and Somatic Genetics since 2013.
CERTIFICATIONS & ACCREDITATIONS
The Biobank Côte d’Azur is certified according to the NF S96-900 standards (quality of Biological Resource Centers). The Biobank was the first pathology laboratory in France to seek and obtain accreditation by COFRAC (ISO 15189 norm,) in 2013, both for pathology and molecular biology activities.

Certification
NF S 96-900

Accreditation n°8-3034
Scope available on
website www.cofrac.fr